Abstract
Introduction:
The 2017 National Comprehensive Cancer Network (NCCN) guidelines for Acute Myeloid Leukemia (AML) recommend performing a bone marrow aspirate (BMA) and trephine biopsy (BMTB) 14-21 days after starting induction therapy (commonly referred to as a "Day 14 [D14] marrow"). Those who do not achieve a hypoplastic marrow, with cellularity <10-20% and blasts <5-10%, are recommended to undergo a second induction cycle (IC). We performed a retrospective analysis to determine the impact of day 14 bone marrow (D14 BM) characteristics in predicting remission, association with overall survival (OS) and the impact of a second IC based on D14 BM results.
Methods:
Patients with AML aged 18-70 years undergoing induction therapy with standard "7+3" regimens at Vancouver General Hospital or University of Alberta Hospital from 2007-2015 and 2005-2015 respectively were included. D14 cellularity was determined on BMTB and blast percentage (blast %) was assessed by morphology on BMA and BMTB. We examined outcomes by three D14 risk categories: 1. blast % (<5/≥5%), 2. cellularity (<20%/≥20%) or 3. blast % and cellularity (< 5% blasts or <20% cellularity/ ≥5% blasts and ≥20% cellularity). Overall response rate was determined at peripheral count recovery and defined as complete remission (CR) or CR with incomplete count recovery (CRi), (Cheson B J Clin Onc 2003; 21(24):4642-9). Differences in categorical variables were determined using Mantel-Haenszel tests. Binary logistic regression was used to determine association between D14 BM and other covariates on CR/CRi rate. Cox regression was used to determine association between covariates with OS.
Results:
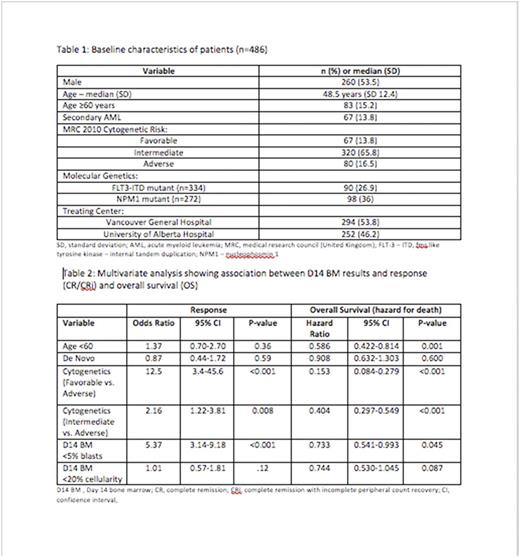
546 patients were identified with 486 (89.0%) undergoing a D14 BM (Table 1). On the D14 BM, 190 patients had blasts ≥5%, 96 had cellularity ≥20% (with 12 patients also having blasts <5%) and 84 had both blasts ≥5% and cellularity ≥20%. The CR/CRi rate stratified by D14 categories was: 87% vs. 56%, p<0.001 (< 5% vs. ≥ 5% blasts), 79% vs. 57%, p<0.001 (< 20% vs. ≥20% cellularity) and 79% vs. 54%, p<0.001 (<5% blasts or <20% cellularity vs. ≥5% blasts and ≥20% cellularity).
In univariate analysis, D14 low-risk categories were associated with higher CR/CRi rates (D14 blasts <5%, OR 5.4, 95% CI 3.5-8.4, p<0.001, D14 cellularity <20%, OR 2.9, 95% CI 1.8-4.6, p<0.001 and D14 blasts <5% or cellularity <20%, OR 3.4, 95% CI 2.1-5.5, p<0.001) and improved OS (D14 blasts <5%, HR 0.66, 95% CI 0.51-0.84, p<0.001, D14 cellularity <20% ,HR 0.55, 95% CI 0.42-0.73, p<0.001 and D14 blasts <5% or D14 cellularity<20% ,HR 0.52, 95% CI 0.39-0.70, p<0.001). In multivariate analysis, the diagnostic cytogenetics and D14 blasts <5% predicted CR/CRi at count recovery whereas age, cytogenetics and D14 blasts <5% were significantly associated with improved OS (Table 2). The risk category using both blast % and cellularity was not included in the multivariate model due to multicollinearity with D14 blast % and cellularity.
131 patients (27%) underwent a second IC, most commonly with etoposide/cyclophosphamide (n=48) or mitoxantrone/etoposide (n=48) regimens based upon the results of the D14 BM. CR/CRi rates for patients receiving 1 vs. 2 ICs in high risk D14 groups were: 43% vs. 62%, p=0.01 (D14 blasts ≥ 5%), 67% vs. 55%, p=0.33 (D14 cellularity >20%) and 38% vs. 55%, p=0.35 (D14 blasts ≥5% and cellularity ≥20%). In patients with ≥5% residual blasts on D14 marrow but <20% cellularity there was significant variation in use of D14 BM results with only 49/106 (46.2%) patients undergoing a second IC. In this group, a second IC improved CR/CRi (43.9% vs. 72.0%, P=0.004) but there was no difference in OS (HR 1.08, 95% CI 0.63-1.86, p=0.77). Furthermore, in this group leukemia free survival following CR/CRi was worse in patients that received a second IC (HR 2.24, 95% CI 1.00-4.98, p=0.05).
Conclusion:
D14 BM results are prognostic for achievement of CR/CRi following induction therapy for AML. D14 blasts <5% predicted CR/CRi in multivariate analysis, despite possible confounding of this analysis by the use of second IC based on D14 BM results. In addition, D14 blasts <5% are prognostic for OS in multivariate analysis including other recognized prognostic factors. For patients with residual blasts but hypocellular marrow on D14 BM, second IC was associated with greater CR/CRi rates but no difference in OS. This finding suggests that for this subset of patients, treatment decisions based on D14 BM may not significantly improve OS.
Hogge: Novartis, Roche, and Sanofi: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal